


Here, the radium-226 nucleus decays into a radon-222 nucleus, freeing an alpha particle simultaneously.īeta decay happens when a neutron is changed over into a proton, which is accompanied by the emission of a beta particle (high-energy electron). An illustration of alpha decay is given underneath. Where, A is the mass number and Z is the atomic number. The condition for alpha decay is: AX Z → A – 4X’ Z – 2 + 4α 2
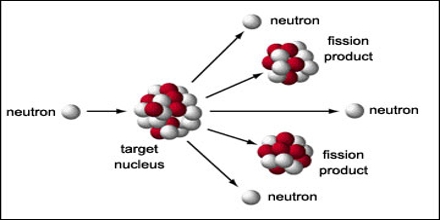

Nuclei with mass numbers more prominent than 200 will quite often go through alpha decay – a cycle where a 4He nucleus, regularly referred to as an alpha particle (42α) is freed from the parent nucleus. Other Important Types of Nuclear Reactions Approximately 1.69*109 KJ of energy is produced for each mole of helium formed. The fusion of deuterium and tritium nuclei is accompanied by a loss of approximately 0.0188 amu of mass (which is totally changed over into energy). These fusion reactions generally happen at the center of the sun and different stars. Subatomic particles, for example, neutrons or protons are also formed as products in these nuclear reactions.Īn example of this reaction is the reaction between deuterium (2H) and tritium (3H) that yields helium (4He) and a neutron (1n). In nuclear fusion reactions, two or more atomic nuclei combine to form one nucleus. One more significant illustration of nuclear fission is the parting of the plutonium-239 nucleus. Different products can be formed from this nuclear reaction, as depicted in the situations underneath. The steam is utilized to pivot turbines in order to generate electricity.Ī significant illustration of nuclear fission is the parting of the uranium-235 nucleus when it is bombarded with neutrons. This is finished by utilizing the heat created from the nuclear reaction to change over water into steam. The energy created from fission reactions is changed over into electricity in nuclear power plants. This reaction was first discovered by the German chemist’s Otto Hahn and Strassmann in the year 1938. These reactions regularly discharge a lot of energy, which is accompanied by the emission of neutrons and gamma rays (photons holding enormous measures of energy, enough to take electrons out of atoms). This process can happen through a nuclear reaction or through radioactive decay. It refers to the parting of an atomic nucleus into two or lighter nuclei. A one gram of matter can release approximately 90,00,00,00,000 kJ of energy. This ‘missing’ mass is changed into energy. To simplify, the products formed in nuclear fission and nuclear fusion generally have a lower mass than the reactants. Nuclear binding energy can be characterized as the energy needed to hold every one of the protons and neutrons inside the nucleus.ĭuring an nuclear reaction (such as a fission or fusion reaction), the mass represented by the nuclear binding energy is delivered as per the condition e = m × c 2 (energy = mass occasions the square of the speed of light). This difference in mass is ascribed to nuclear binding energy (frequently alluded to as a mass defect). The mass of an atomic nucleus is less than the amount of the masses of each subatomic particle combined that constitutes it (protons and neutrons). Nuclear Reactions Releasing Tremendous Amounts of Energy Nuclear fusion reactions are the cycles where two somewhat light nuclei (by means of a crash) manage a single, heavier nucleus. The previous includes the retention of neutrons (or other generally light particles) by a heavy nucleus, which makes it split into (at least two) lighter cores. Two prominent types of nuclear reactions are nuclear fission reactions and nuclear fusion reactions.
#Nuclear fission example how to#
How to Connect Python with SQL Database?.ISRO CS Syllabus for Scientist/Engineer Exam.ISRO CS Original Papers and Official Keys.GATE CS Original Papers and Official Keys.


 0 kommentar(er)
0 kommentar(er)
